Prof. Dr. Jeffrey A. Reimer, University of California Berkeley, Dept. of Chem. and Biomolecular Engineering

Jeffrey A. Reimer received his bachelor’s degree (with honors) from the University of California at Santa Barbara, and his doctorate from the California Institute of Technology. After his postdoc at IBM Research in New York he joined the Berkeley faculty where he is presently the C. Judson King Endowed Professor and the Warren and Katharine Schlinger Distinguished Professor and Chair of the Chemical and Biomolecular Engineering Department. Professor Reimer has won the UC Berkeley Distinguished Teaching Award, the highest award bestowed on faculty for their teaching. His research generates new knowledge for environmental protection, sustainability, and provides fundamental insights to condensed matter via materials chemistry, physics, and engineering. He is recognized for these works by election as a Fellow of the American Association for the Advancement of Science, a Fellow of the American Physical Society, and a Fellow of the International Society for Magnetic Resonance. In 2015 he received a Research Fellowship from the Alexander von Humboldt Foundation. In addition to his ~225 research publications, Professor Reimer is co-author (with T.M. Duncan) of the introductory text Chemical Engineering Design and Analysis, and the text Carbon Capture and Sequestration.
Our Changing Atmosphere: Evidence that demands a Verdict
Many students and colleagues are only aware of climate change by way of public discourse and social media. Drawing on recent scientific papers organized for a course he teaches at Berkeley, Jeffrey Reimer will show how the atmosphere is changing, that humans are the cause, and that there are consequences. These consequences may be viewed in the context of Earth’s historical carbon cycles, which demonstrate well what the Earth will look like unless we consider every possible means to decarbonize the atmosphere.
Prof. Dr. Johannes A. Lercher, Technical University of Munich, Chemical Technology

Sorption and catalysis at the liquid-solid interface
Johannes A. Lercher
Institute for Integrated Catalysis, Pacific Northwest National Laboratory, Richland, WA
Department of Chemistry Technische Universität München, Garching, Germany
Characterizing, understanding, and controlling the interactions and catalytic transformations at solid-liquid interfaces is complex and experimentally as well conceptually challenging. The lecture will describe potential approaches to characterize the impact of protic and non-protic solvents at that interface and in particular in presence of water. The impact of the solvent on properties of metal oxides, zeolites and supported metal particles as well as on the interacting molecule will be discussed and quantified in terms of the excess chemical potentials. Examples discussed will include adsorption and reactivity of organic molecules, H2 and CO on oxides, zeolites and metal particles. Opportunities and challenges for acid-base and redox catalysis with oxides and metals with and without external electric potentials will be discussed.
Prof. Dr. Umit S. Ozkan, The Ohio State University, Dept. of Chem. and Biomolecular Engineering

Umit S. Ozkan is a Distinguished University Professor at The Ohio State University. She received her Ph.D from Iowa State University in 1984 and joined the faculty of The Ohio State University in 1985. Between 2000 and 2005, she also served as the Associate Dean for Research in the College of Engineering. She held Visiting Scientist and Visiting Professor positions at the French Institut de Recherches sur la Catalyse (Catalysis Research Institute) Centre National de la Recherche Scientifique (CNRS) and Université Claude Bernard, respectively. Currently, she is the Chair of the Chemical and Biomolecular Engineering Department.
Dr. Ozkan’s current research interests are focused on heterogeneous catalysis and electro-catalysis. She has edited eight books, has written over 250 refereed publications and book chapters, given over 350 conference presentations and over 150 invited lectures in 20 different countries. She has seven patents and over 12,000 citations. Professor Ozkan has held and continues to hold many leadership positions in several professional organizations. She served as the Co-chair of the Continuing Symposia in Catalysis for the Colloids and Surface Chemistry Division (1994-2000), the ACS Petroleum Chemistry Division Secretary (1998-99), member of the Board of Directors of the Catalysis and Reaction Engineering Division of AICHE (1996-1999, 2008-2011). In 2002-2003, she served as the President of the ACS, Petroleum Chemistry Division. She was the Secretary for the North American Catalysis Society (2000-2009) She is on the Editorial Boards of Catalysis Today, Journal of Molecular Catalysis, Catalysis Letters, Topics in Catalysis, The Royal Society of Chemistry, Catalysis Book Series, Applied Catalysis B, ACS Applied Energy Materials, Catalysis Reviews in Science and Engineering, ACS Catalysis, Nature Sustainability, and Journal of Catalysis. Dr. Ozkan is a Professional Engineer registered in Ohio. She is a fellow of the American Association for the Advancement of Science (AAS), American Institute of Chemical Engineers (AICHE), and American Chemical Society (ACS).
Professor Ozkan is the recipient of many honors and awards among which are ACS Henry H. Storch Award (2017), ACS Energy and Fuels Distinguished Researcher Award (2012), John van Geuns Lectureship Award at the Van’t Hoff Institute at the University of Amsterdam (2010), Iowa State University, Professional Achievement Citation in Engineering (2010), AICHE Mentorship Excellence Award (2009), Fulbright Senior Scholar Award (2007), OSU College of Engineering Scott Faculty Excellence Award (2004), the Society of Women Engineers Achievement Award (2002), American Chemical Society, Columbus Section Outstanding Research in Chemistry Award (2002), the Ohio State University Distinguished Scholar Award (1999), Iowa State University, College of Engineering Professional Progress Award (1999), Pittsburgh-Cleveland Catalysis Society Award (1998), French C.N.R.S. Fellowship (1994-95), Keck Foundation Excellence in Engineering Education Award (1994), OSU College of Engineering Harrison Outstanding Faculty Award (1993), OSU College of Engineering Lumley Research Award (1991, 1996, 2000, 2006, and 2011), National Science Foundation Woman Faculty Award for Excellence in Teaching and Research (1991), OSU College of Engineering McQuigg Outstanding Teaching Award (1990), and Union Carbide Innovation Recognition Award (1991, 1992). In 2013, a special volume of Topics in Catalysis, a premier journal in the field of catalysis, was dedicated in her honor (Volume 56, issues 18-20). The volume included contributions from 35 different research groups from 12 different countries. In 2019, she was again honored, this time by a special volume of Catalysis Today (Volume 323, 270 pages, 2019).
In her research group, Dr. Ozkan has advised and mentored over 100 graduate students, post-doctoral researchers and honors students.
High-temperature Co-electrolysis of CO2 and H2O on Lanthanum Ferrite-type Perovskite Oxide Cathodes
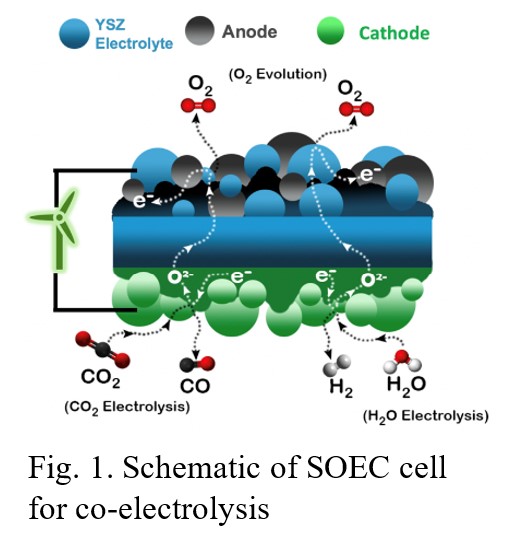
High-temperature co-electrolysis of H2O and CO2 is a promising way to produce clean syngas which is an important chemical building block. This scheme provides a potential CO2 mitigation route by converting this greenhouse gas into a valuable product, with pure oxygen being the only by-product. In this study, a high-temperature (700-850°C) solid electrolysis cell (SOEC) is used to co-electrolyze H2O and CO2 into syngas.
The SOEC employed in the present work consists of an yttria-stabilized zirconia (YSZ) solid oxide oxygen ion conducting electrolyte, sandwiched between two electrode layers (Fig. 1). The counter electrode (anode) is a commercially available mixture of La0.8Sr0.2MnO3 and YSZ (LSM-YSZ), whereas the working electrode (cathode) consists of an in-house developed lanthanum ferrite-type perovskite oxide. H2O and CO2 get electrolyzed to H2 and CO at the cathode producing O2- ions, which travel through the YSZ electrolyte to the anode where they combine to form molecular oxygen.
The A- and B-site doped lanthanum ferrite perovskite materials used in this study as the working electrode were synthesized via EDTA-citric acid complexation method. The synthesized materials were characterized ex-situ, in-situ and operando using X-ray diffraction (XRD), photoelectron (XPS) and absorption (XAS) and Raman spectroscopy, transmission electron microscopy and four probe DC van der Paw techniques to investigate their morphology, bulk and surface structure, electrical conductivity and the changes in these properties during the electrochemical reaction under bias. The electrocatalytic activity tests showed that the H2/CO ratio in the product stream can be tuned through modifications on the A- and B-site doping as well as other reaction parameters.

